
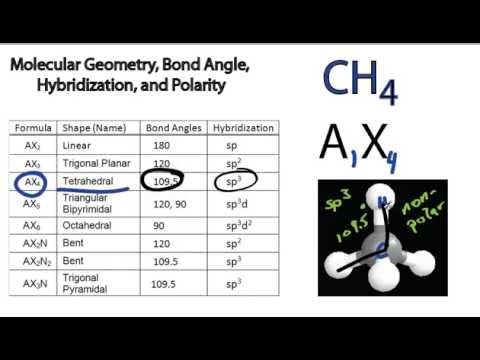
Indeed, it is helpful in organic chemistry and biochemistry as a model of the effects of various substituents on the geometry of the carbon atom. The molecular geometry of methane (CH) is of interest to researchers in many fields.

This means that the two atoms forming the molecule are connected by a bond that is not a simple straight line. It is the simplest example of a homonuclear diatomic molecule that is non-linear. It exists at low concentrations as a gas in Earth’s atmosphere. The molecule H2 is a diatomic molecule with the chemical formula H2. What is the molecular geometry of ch4?īecause, according to VSEPR theory, molecular geometry considers only bond pairs or atoms, while electron geometry considers bonded atoms and lone pairs present on the central atom, CH4’s molecular geometry and electron geometry are tetrahedral. The three bonds meaning away from the apex are shorter than the three bonds pointing towards the apex. The apex represents the central atom’s lone electron pair. The three bonds pointing towards the apex are the axial bonds. The three bonds meaning away from the height, are the equatorial bonds. The central atom (the pyramid’s base) has three bonds pointing towards the apex and three bonds away from the peak. A trigonal pyramidal molecular geometry is a molecular geometry. Davy had previously isolated the element oxygen in 1795.Ĭlo2 has a molecular geometry of CClF, which is a trigonal pyramidal molecular geometry. Clo2 was the first ionic substance prepared artificially by the English chemist Sir Humphry Davy in 1808. The combination is a colourless, volatile helpful liquid as a bleach and dioxygen source in chemical reactions. What is the molecular geometry of clo2?Ĭlo2, also known as dioxygenyl dichloride, is a chemical compound with the formula ClO2. Two different atoms are bonded at each of two opposite corners. Chloroethane (CH4) and hydrocyanic acid (HCN) have trigonal pyramidal structures these molecules have three bonds running between each pair of atoms, creating an overall pyramid shape. Chlorine trifluoride (ClF3) has a linear molecular geometry with three unpaired electrons in its outer shll.īrmine pentafluoride (BrF5) has a linear structure with five unpaired electrons on its outer surface. This molecule also has two unpaired electrons. Hydrogen sulphide (H2S) has a linear molecular geometry, meaning that its molecular bonds run along one straight line. With that in mind, fluorine would want to approach two other fluorines as closely as possible for maximum bonding capability. The goal of bonding is to get as many shared pairs as possible. It has three pairs of unshared valence electrons outside its outermost shell. So when we look at ClF3, it’s a highly electronegative fluorine that determines where its electrons will be on Cl-F-Cl bonds. In the VSEPR view, we use electron bonds to describe how valence electrons are distributed around an atom in a molecule. The bond angle in any molecule can be described using VSEPR theory. Hydrogen Chloride is bent because two different particles and three bonds surround the central atom. They are not linear because the central atom is surrounded by two other atoms and two other bonds. Chlorine, Chlorine Dioxide, and Bromine are all cyclic. I2 and BrF3 are both linear, but BrF3 is bent. Some molecules are linear, some are cyclic, and some are bent. The shape of a molecule can tell you a lot about how a molecule will behave. We will look only at the molecular systems of some common molecules throughout this blog post. Conclusion: What is the molecular geometry of h2s, clf3, brf5, clo2, ch4, h2co, icl2 and bro3?.Stability Of The Periodic Table Trend In Group 17 Elements.Melting & Boiling Points For Group 17 Elements.Physical Properties of Group 17 Elements.What is the molecular geometry of bro3?.What is the molecular geometry of icl2?.What is the molecular geometry of h2co?.What is the molecular geometry of clo2?.What is the molecular geometry of brf5?.What is the molecular geometry of clf3?.What is the molecular geometry of h2s, clf3, brf5, clo2, ch4, h2co, icl2 and bro3?.


 0 kommentar(er)
0 kommentar(er)
